|
Elena Orlova
Katie Reader |
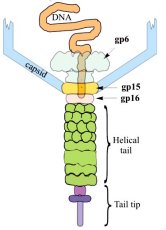
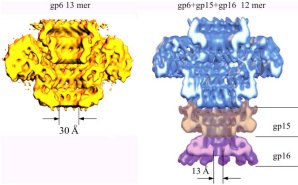
Structure of a viral DNA gatekeeper at 10 Å resolution by cryo-electron microscopyThe structure of the connector highlights the function of this multi-protein complex as a gatekeeper of the packaged viral DNA. The connector of Bacillus subtilis bacteriophage SPP1 is composed of the portal protein gp6 (subunit molecular mass of 57.3 kDa) and the two head completion proteins gp15 (11.6 kDa) and gp16 (12.5 kDa). The portal protein participates in the early reactions of procapsid assembly. Although the portal protein alone is responsible for DNA packaging into the capsid, attachment of gp15 and gp16 to the portal vertex after termination of DNA encapsidation is required to retain the packed DNA inside the capsid. The connector represents an intermediate in assembly of the phage head-to-tail interface, whose function is to prevent DNA leakage immediately after the packaging reaction. The phage tail attaches to the gp16 ring whereas the DNA extremity, which is packaged last, remains attached to the connector structure. Initiation of phage infection requires the opening of the connector to enable the release of the viral chromosome through the tail channel into the host cytoplasm. The ubiquity of the portal vertex in tailed bacteriophages and likely in herpes viruses strongly suggests that a number of the molecular mechanisms discussed here, essential for SPP1 assembly, apply also to many viruses. We have performed structural analysis by cryo-electron microscopy and angular reconstitution of the ~900 kDa connector complex and of the portal protein. Maps at ~10 Å and ~9 Å resolution achieved for the connector and for the portal protein revealed many interesting details. Comparison of the structures of gp6 from the connector complex and of assembly-naïve gp6 shows that the portal protein wings and crown are stable although the stem undergoes noticeable conformational changes. The conformational change at the bottom of the stem that leads to exposure of the gp6 interface for interaction with gp15 is most likely concomitant with termination of DNA packaging. The connector structure shows that gp15 serves as an extension of the portal protein channel where gp16 binds. The central channel is closed by gp16 physically blocking the exit from the DNA-filled capsid. Comparison of portal protein structures from different phages exhibits their common structural organisation. Conservation of the structural elements lining the central channel and their similar dimensions might suggest association with a common function in DNA packaging. Other portal structure domains show more variable structure and size (e.g. large wing and crown domains in gp6). |
|
EM group web page
Elena Orlova |