
Additional material for the papers by Roseman et al (1996) Cell 87, 241-251
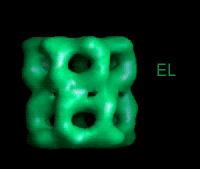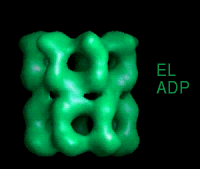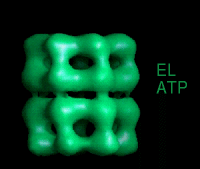
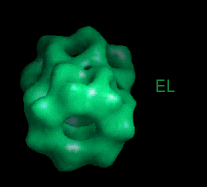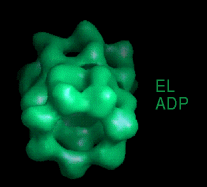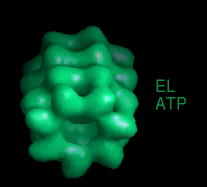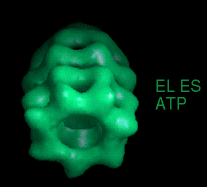
These movies are short sequences of GroEL structures in complex with different nucleotides and GroES, created from surface-rendered images of cryo EM 3D reconstructions. They should be viewed as a repeating loop, and give the impression of domain movements occurring during the ATPase cycle. The letter codes are EL, GroEL; EL ES, GroEL-GroES. The movies should be viewed in 256-color or full colour mode.
 |
This sequence shows the GroEL movements seen directly from the side (i.e., perpendicular to the 7-fold axis of the oligomer). |
 |
The molecule is tipped forward to allow a view into the cavity of the top ring. The domain rotation caused by nucleotide binding continues into the large movement accompanying GroES binding. |
 |
The sequence of 4 states is shown tipped backwards, revealing a different pattern of movement in the lower ring. The upper ring (at the back of the images) performs a continuous twisting motion, but the lower ring opens and shuts with radial movements of the domains in addition to the twisting. |
Additional material for the paper by Rye et al (1999) Cell 97, 325-338
The following movies show GroEL-GroES complexes alternating between ATP (T) and ADP (D) forms. The GroES binds to the two rings in alternation.
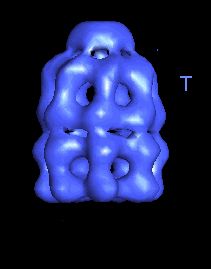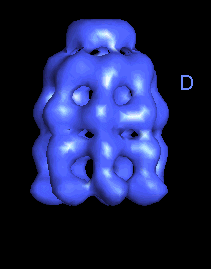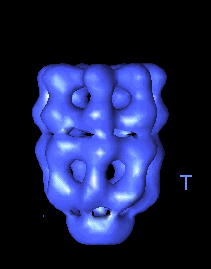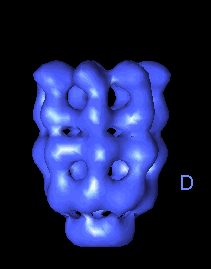 |
This movie shows the alternation of GroES binding by flipping between "up" and "down" structures. |
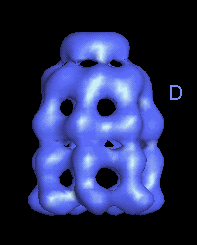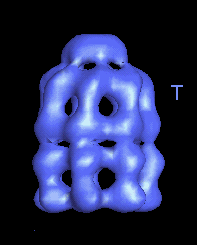 |
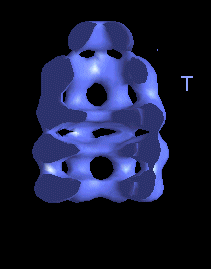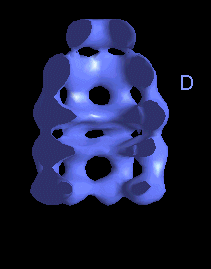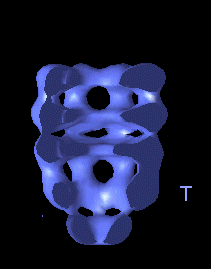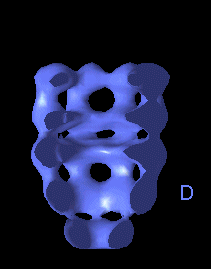
This movie is shown without flipping to illustrate the conformational changes between ATP and ADP forms. |
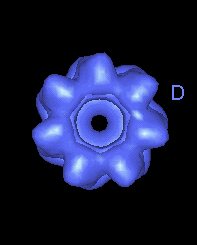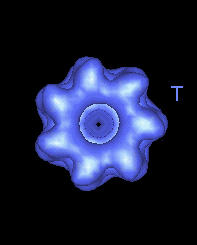 |
This movie shows the movements at the open end of the complex (remote from GroES). |
 |
The structure is cut open to reveal the binding cavities and show their movements. |
Back to Birkbeck Chaperone page
Link to Birkbeck Crystallography EM and Image Processing group