Amyloid Assemblies
Crystallography, ISMB
Birkbeck College London |
 |

Helen Saibil
Jonathan O'Driscoll
Former group members
Silvia Panico (Imperial College)
Lilia Milanesi (Queen Mary College)
Jose Jimenez (Sanger Centre)
Julie Hodgkinson (Medizinische Hochschule Hannover)
Sara Cohen-Krausz made
important contributions to our amyloid work, and sadly lost her battle with cancer in May 2009.
After her stay at Birkbeck, she worked in the Hebrew University, Jerusalem, until her untimely death. |

|
Amyloid fibrils are insoluble aggregates that result from the self-assembly of partially unfolded proteins. Regardless of the native structure of the precursor proteins, the predominant secondary structure in the fibrillar form is beta sheet. Proteins that form amyloid in vivo are associated with diseases such as Alzheimer's and CJD.
SH3 Amyloid (Jimenez et al.) |
|
In collaboration with Dr Margaret Sunde (now at University of Sydney,
Australia) and
Prof Chris Dobson
,
we determined the low resolution structure of amyloid fibrils formed from a SH3 domain by cryo-electron microscopy and image processing (Jimenez, J.L., Guijarro, J.I., Orlova, E., Zurdo, J., Dobson, C.M., Sunde, M. and Saibil, H. (1999) Cryo-electron
microscopy structure of an SH3 amyloid fibril and model of the molecular packing.
EMBO J 18, 815-821).
Further work was published in: Dependence on solution conditions of aggregation and amyloid formation by an SH3 domain. Zurdo J, Guijarro JI, Jimenez JL, Saibil HR & Dobson CM (2001) J Mol Biol. 311, 325-340. |
 |
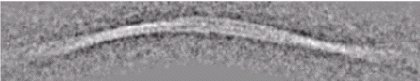
Cryo EM image of SH3 fibrils:
The fibrils are hollow and contain two pairs of protofilaments twisting around each other. |
 | Cross-section of the fibril, showing four protofilaments surrounding a hollow core. A surface view of the 3D map is shown on the left. |
 |
In a schematic molecular model based on the EM density, the core of each protofilament is formed of two beta-sheets. The regions linking the protofilament cores are less compact and/or more disordered. These loop regions are represented by spheres to indicate the number of residues in the loops. |
 |
The protofilaments are thin and flat, so that the beta-sheets must be nearly flat. The cross-beta part of the model is shown inside the EM density (transparent blue surface). Flat beta sheets can be found in the PDB, for example, in the alkaline proteases. |
Insulin amyloid fibrils (Jimenez et al.)
The polypeptide hormone insulin consists of two chains linked by
disulfide bonds. The native structure is almost completely alpha-helical,
but denatured insulin readily forms amyloid fibrils, which requires
a complete refolding of the insulin chains.
In collaboration with Professors
Chris Dobson and
Carol Robinson,
University of Cambridge,
we have used cryo EM to characterise several different forms of insulin amyloid fibrils (The protofilament structure of insulin amyloid fibrils.
Jimenez, JL, Nettleton, E, Bouchard, M, Dobson, CM, Robinson,
CV & Saibil, HR (2002) Proc. Natl. Acad. Sci. USA 99, 196-201). |
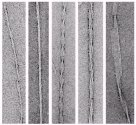 |
Negative stain EM images of insulin fibrils, showing some of the diverse fibril morphologies found in the samples. |
 |
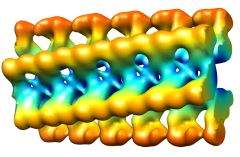
3D maps of 4 different insulin fibril morphologies. The distance between helical crossovers is around 100 nm. The fibrils are made up of different numbers of component strands (protofilaments; from left to right: 2,4,6,and probably 6). The protofilaments are twisted around eachother in either compact or ribbon-like arrangements. |
 |
A molecular model of the compact, 4-protofilament insulin fibrils.
|
Ex vivo lysozyme fibrils (Jimenez et al.)
In collaboration with
Prof. Mark Pepys and
Dr Glenys Tennent at the National Amyloidosis Centre,
Royal Free and University College Medical School,
we have examined lysozyme fibrils isolated from pathological tissue.
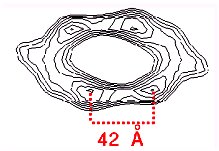
The fibrils have a wavy shape. An averaged repeat from cryo EM images and a schematic 3D model in side view and an end view of the spiral model with 6 protofilaments are shown below.
(Structural diversity of ex vivo amyloid fibrils studied by
cryo-electron microscopy. (2001) Jimenez, JL, Tennent, G, Pepys,
M & Saibil, HR, J. Mol. Biol. 311, 241-247).
|
Mammalian Prion fibrils (Tattum et al.)
In collaboration with
Profs. John Collinge and Tony Clarke at the MRC Prion Unit, Institute of
Neurology, University College London
we have examined PrP fibrils formed in vitro. |

| Elongated oligomers
assemble into mammalian PrP amyloid fibrils. Tattum et al (2006) J Mol Biol 357,
975-985.
|
Beta-2 microglobulin amyloid
In collaboration with
Prof. Sheena Radford at Leeds University
we are studying the 3D structure and properties of beta-2 microglobulin amyloid fibrils. |

| Globular tetramers of beta-2 microglobulin assemble into elaborate amyloid fibrils. White et al (2009) J Mol Biol 389,
48-57.
|
Yeast Prions
In collaboration with
Prof. Achilleas Frangakis at University of Frankfurt and Jens Tyedmers at ZMBH, Heidelberg
we are studying yeast prion assemblies. This work was started during a sabbatical at EMBL Heidelberg. |